Data Processing & Privacy

HEAL-COVID Data Processing Website Statement
HEAL-COVID is a research study comparing different treatments to see which are better at preventing and treating the long term symptoms of COVID-19. If you have agreed to take part in HEAL-COVID, or are considering taking part, you will have received the study information sheet. The information sheet contains all the key details about study involvement and if you have any questions about this you should ask the doctors and nurses looking after you, or who have invited you to take part.
On this page you can find more detailed information about how the HEAL-COVID team are using your data, who will have access to information about you, and who to contact if you have any questions about how your data is handled.
Who is running the study?
Cambridge University Hospitals NHS Foundation Trust and The University of Cambridge jointly act as the Sponsor of this study and are responsible for managing it. They are based in United Kingdom. They have asked that the day today running of the study is carried out by a team based at the Liverpool Clinical Trials Centre (LCTC, part of the University of Liverpool),researchers from The University of Cambridge and researchers from the Centre for Health Economics and Medicines Evaluation (CHEME), part of Bangor University(the central study team). The central study team will need to use information from you and from your healthcare records for this research project.
Cambridge University Hospitals NHS Foundation Trust,The University of Cambridge and the University of Liverpool are the joint data controllers for HEAL-COVID,and are responsible for looking after your information and using it properly.
Data protection regulation requires that we state the legal basis for processing information about you.In the case of this study, we are using your Personal Data for research purposes, and this is ‘a task in the public interest’. Some Personal Data is considered sensitive and is called “special category”. This includes data concerning physical or mental health conditions, which is collected for HEAL-COVID. To process this we rely on the legal basis that “processing is necessary for archiving purposes in the public interest, scientific or historical research purposes or statistical purposes”. You can read more about personal and sensitive data on the LCTC website.
The study has been reviewed by the Medicines and Healthcare Products Regulatory Agency(MHRA), the Health Research Authority and the National Research Ethics Service Committee to make sure that the study is scientifically and ethically acceptable. This study is funded by the National Institute for Health Research Health (NIHR; study identifier 133788)and the NIHR Cambridge Biomedical Research Centre.
The central study team are working with a company called Aparito, based in the UK, to produce the mobile/tablet app that you will complete your follow-up questionnaires on, if you have chosen to take part in this optional follow-up.
What information is collected about me?
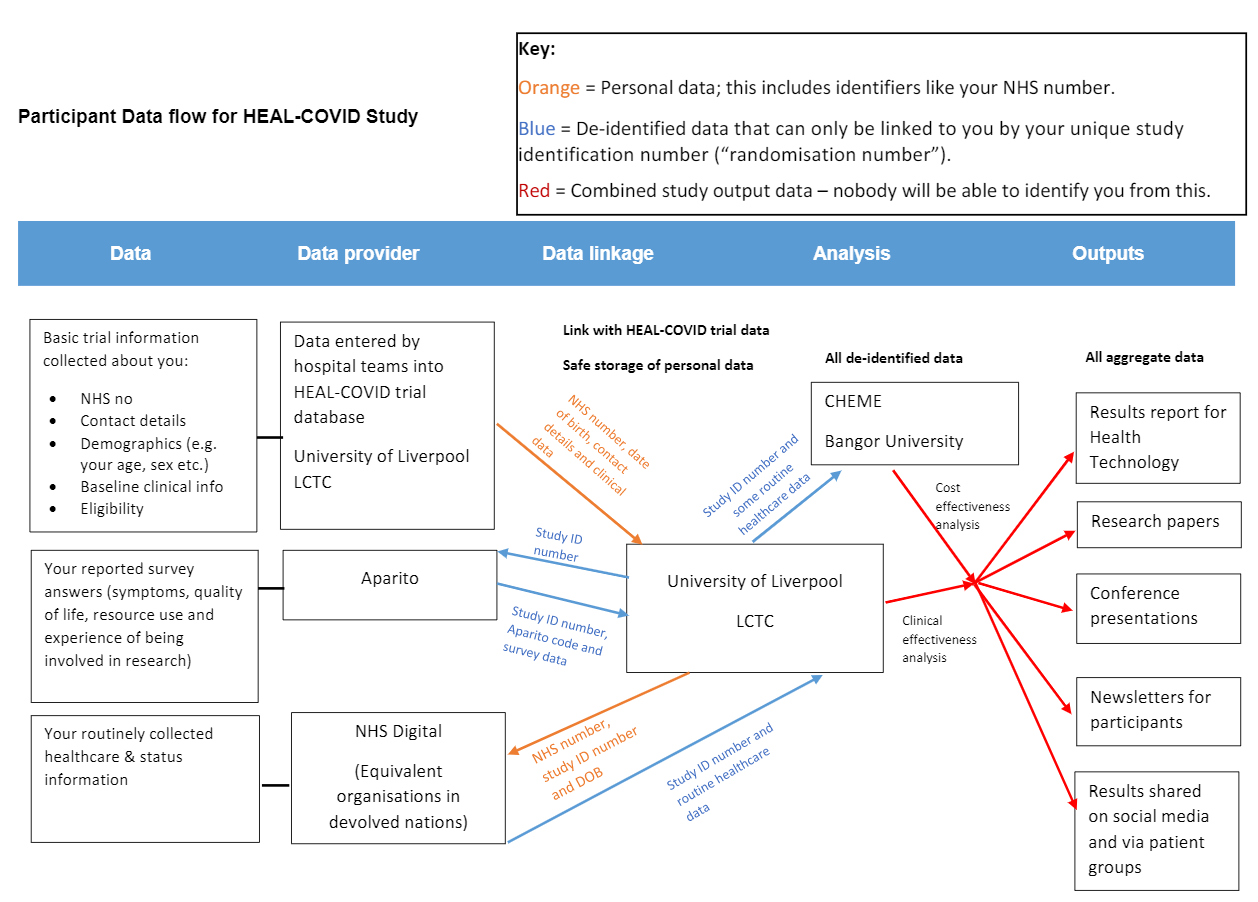
Staff from your hospital team will enter information from your medical records onto the study database. This will include some medical/healthcare information about you, as well as your name, NHS number(CHI in Scotland or Health & Social Care number in Northern Ireland), date of birthand contact details. Your identifiers will be encrypted to protect them. All information collected about you will be used to do the research or to check your records to make sure that the research is being done properly.
In HEAL-COVID we will also collect information about your GP appointments, referrals,hospital visits or other healthcare over the 12 months after you agree to take part in the study. This information is routinely collected by NHS Digital in England, and equivalent organisations in Scotland, Wales and Northern Ireland. To access your healthcare data, LCTC will send details including your NHS number/CHI number/Health & Social Care number, date of birth and unique study participation number to NHS Digital or the equivalent organisations in Scotland, Wales and Northern Ireland in order for them to identify your routine healthcare records. NHS Digital or equivalent will return your routine data to LCTC with your NHS/CHI number removed, so that the details of your healthcare are linked to you only by your unique study participation number.
We plan to collect this data for 12 months from the day that you join the study. In future this might be extended. We may also link your information to other data sources, including databases from other clinical trials, to answer clinically relevant questions.If new data sources are identified or the data collection period is updated, we will include this on this web page.
Participant Data flow for HEAL-COVID Study
Who will have access to my information?
All the confidential information about your participation in this study will be kept confidential. People who do not need to know who you are will not be able to see your name or contact details. Your data will have a unique code number instead.
As well as the central study team, regulatory organisations and auditors or inspectors on behalf of the Sponsor organisations may need to look at your medical and research records to check the accuracy of the research study.
If you agree to take part in the optional follow-up questionnaires via our smartphone/tablet app, Aparito (the company who have made the HEAL-COVID app) will be able to see any information you enter when completing these. They will not know your name or personal details, or have access to your routine healthcare records. Aparito will collect your IP address, which is a unique label identifying the phone or tablet that you complete your questionnaires on.
We will keep all information about you safe and secure. Once we have finished the study, we will keep the data for 10 years, so we can check the results. We will write our reports in a way that no-one can work out that you took part in the study.
What are my choices about how my information is used?
You can stop being part of the study at any time, without giving a reason, but we will keep information about you that we already have.If you no longer wish to take part in HEAL-COVID you should speak to your local hospital team via the contact details on the information sheet you were given when you agreed to take part.
If you choose to stop taking part in the study, we would like to continue collecting information about your health from routine healthcare records. If you do not want this to happen, tell us and we will stop.
In some cases, however we may need to continue to collect limited information about any side-effects of the study treatment you may experience. We will only do this where we are required to do so by law.
We need to manage your records in specific ways for the research to be reliable. This means that we won’t be able to let you see or change the data we hold about you.
Information sharing for other research
When you agree to take part in a research study, the information about your health and care may be beneficial to researchers running other research studies in this organisation and in other organisations. These organisations may be universities, NHS organisations or companies involved in health and care research in this country or abroad. Your information will only be used by organisations and researchers to conduct research in accordance with the UK Policy Framework for Health and Social Care Research.
If you agree to take part in this study, you will have the option to take part in future research using your data saved from this study.
Where can I find out more about how my information is used?
You can find out more about how we use your information and your rights:
- on the LCTC website, https://lctc.org.uk/privacy
- at www.hra.nhs.uk/information-about-patients
- in the Health Research Authority leaflet available from www.hra.nhs.uk/patientdataandresearch
- by contacting the Cambridge University Hospitals NHS Foundation Trust Data Protection Officer on infogov@addenbrookes.nhs.uk
- by contacting the University of Cambridge Data Protection Officer on dpo@admin.cam.ac.uk
- by contacting the University of Liverpool Data Protection Officer on legal@liverpool.ac.uk
- by asking one of the research team at your hospital
Can I complain?
Yes. If you are not happy with the way your information is being handled, or with the response received from us, you have the right to lodge a complaint with the Information Commissioner’s Office at Wycliffe House, Water Lane, Wilmslow, SK9 5AF (www.ico.org.uk).